The amount of solar energy falling on the earth is 10,000 times more than the energy consumed by all humans
Solar energy is a never-ending energy that falls on the earth as long as the sun exists. According to data from a while back, humans consume 18 TW (terawatts) of energy in a year, while 180,000 TW of solar energy flows onto the earth. It is weakened by scattering in the atmosphere, and large part of it cannot be used realistically, such as the quantity that falls onto the middle of the Pacific Ocean or the summit of Mount Everest, but it is still possible to use about 1,000 TW based on calculation.
Still, the density of solar energy is low. If you try to collect a lot by using solar panels, large areas are required. The biggest drawback is cost. I think many people will imagine solar power generation when thinking of technology using solar energy, but it is expensive. The current technology achieves energy conversion efficiency exceeding 30%, but it is still not widespread owing to economic difficulties.
On the other hand, research on artificial photosynthesis is also progressing. Photosynthesis in plants uses solar energy to produce sugars from water and CO2. Because this sugar has higher energy than the source water and CO2, it can be considered as storage of solar energy. The chemical reaction that uses solar energy to produce other compounds with higher energy from water is called artificial photosynthesis. Artificial photosynthesis is a phenomenon that can occur using photocatalysts that absorb light and promote chemical reactions. For example, if photocatalyst powder is placed in water and exposed to light, water can be decomposed to produce hydrogen and oxygen without electrodes.
It was Japanese scientists who discovered the basis of artificial photosynthesis. It was common knowledge in electrochemistry that the electrolysis of water could only occur if a certain voltage was applied between the two electrodes. However, in 1972, it was reported that when titanium dioxide (TiO2) and platinum (Pt) electrodes were used, water decomposition occurred when the TiO2 electrode was exposed to light, resulting in the generation of oxygen and hydrogen. The phenomenon is called the Honda-Fujishima Effect after the discoverers.
It was just around time of the oil shock in the 1970s, and research into the use of solar energy was being promoted worldwide, but artificial photosynthesis was far from practical in use, and researchers in Japan and abroad terminated their research one after another. The photocatalysts under study were mainly used for environmental purification, and products for the anti-fogging and anti-smudging of glass and the like were released, so I guess they are more familiar to you than artificial photosynthesis. After that, interest died down in Japan as well.
However, as a result of continued assiduous research, high-efficiency water decomposition using ultraviolet light was achieved around the year 2000. In the mid-2000s, water decomposition using visible light, which has less energy than ultraviolet light, was also achieved. Research on artificial photosynthesis began to flourish again. Since then, it has been demonstrated with actual sunlight, and after 2010, it became possible to calculate the energy conversion efficiency in the case of using sunlight.
The challenge of solar power generation is cost, while the challenge of artificial photosynthesis is efficiency
Artificial photosynthesis has potential in terms of cost, which is a problem with solar power generation.
In solar cells, when sunlight hits, negative and positive electricity are generated and separated into different electrodes. In other words, it converts solar energy into electric energy, but to store it, you need a storage facility such as a large battery, and natural discharge occurs. Also, in the case of converting solar energy into hydrogen energy, which is easier to store than electricity, you need to perform two steps; first convert it into electric energy and then use the energy to electrolyze water.
Artificial photosynthesis, on the other hand, can convert solar energy directly into hydrogen. If many steps are involved, a system will inevitably become cumbersome and there will be economic problems, so it is a great advantage that it can be simplified.
On the other hand, the challenge of artificial photosynthesis is the efficiency of how well photocatalysts work.
There are two main types of photocatalysts. One is in the form of a molecule formed by a collection of elements, such as pigments that absorb light and cause a reaction. Another is a compound in which more atoms come together and can be recognized as a solid. The TiO2 mentioned above falls under this type, and when it comes to personal items, fine particles contained in toothpaste used to produce whiteness and gum are examples of it.
Even compounds that act as photocatalysts generate negative and positive electricity when exposed to light, leading to a reduction reaction that gives electrons to the reactants and an oxidation reaction that draws electrons from the reactants. However, it is actually easier to go back to the original state without splitting if there are conflicting reactions in the very small area of particles. In other words, when we generate negative and positive electricity, it does not leave the photocatalyst and often sticks again.
To overcome these problems, our laboratory is conducting research with the aim of developing new photocatalyst materials and improving the efficiency of photocatalysts.
There are two guidelines for improving photocatalyst efficiency. The first point is to absorb a wide range of different wavelengths of light contained in sunlight. The second point is to use the absorbed solar energy for chemical reactions while minimizing waste.
The longer the wavelength of light, the weaker the energy. For example, ultraviolet light, which has a short wavelength, has strong energy, so the chemical reaction proceeds even if a fraction is lost, but the chemical reaction proceeds less for visible light and infrared light with weaker energy.
The wavelength at which a photocatalyst can absorb energy depends on the material, so it depends on the possibility of finding a new material or making a good compound. We can achieve artificial photosynthesis if energy can be used in near-infrared light without loss, but it is quite difficult in reality. Currently, our goal is to utilize ultraviolet and visible light as efficiently as possible.
If artificial photosynthesis can be implemented in society, even the concept of using hydrogen as a raw material will be changed
While hydrogen is known as a clean energy source that does not emit CO2 during combustion, what about when it is made? Hydrogen is classified into three colors based on how it is produced.
Traditionally, fossil fuels have been the source of hydrogen. The commonly used method is called steam reforming. It is produced by reacting water with hydrocarbons such as methane contained in natural gas, and is also called gray hydrogen because CO2 is emitted during the reaction.
On the other hand, even if the same fossil fuel is used, a hydrogen production method is currently being considered, which is performed by burying CO2 deep in the ground so that it does not enter the atmosphere. The hydrogen produced by this method is called blue hydrogen. However, burying CO2 will only postpone the problem and is not a fundamental solution.
Ideally, CO2 should not be emitted, and this is exactly what artificial photosynthesis can achieve. Hydrogen produced by water decomposition using solar energy is called green hydrogen.
In the first place, hydrogen has a wide range of uses other than power generation. The synthesis of ammonia used in fertilizers from hydrogen and nitrogen in the air was reported as a major discovery in catalyst chemistry about 100 years ago. It was such an achievement that it is said that there could not have been so many people on earth without that discovery. Also, synthetic gas, which is a mixture of hydrogen and carbon monoxide, can produce chemical products. It is also very important as a raw material for the chemical industry.
But what if we could make those reactions happen without hydrogen? In artificial photosynthesis using water as a raw material, CO2 can be reduced to produce a raw material for chemical products, and nitrogen can be reduced to ammonia. Then, even the concept of turning hydrogen into a raw material will be overturned. That would be a huge benefit for humanity because it would reduce the cost of manufacturing.
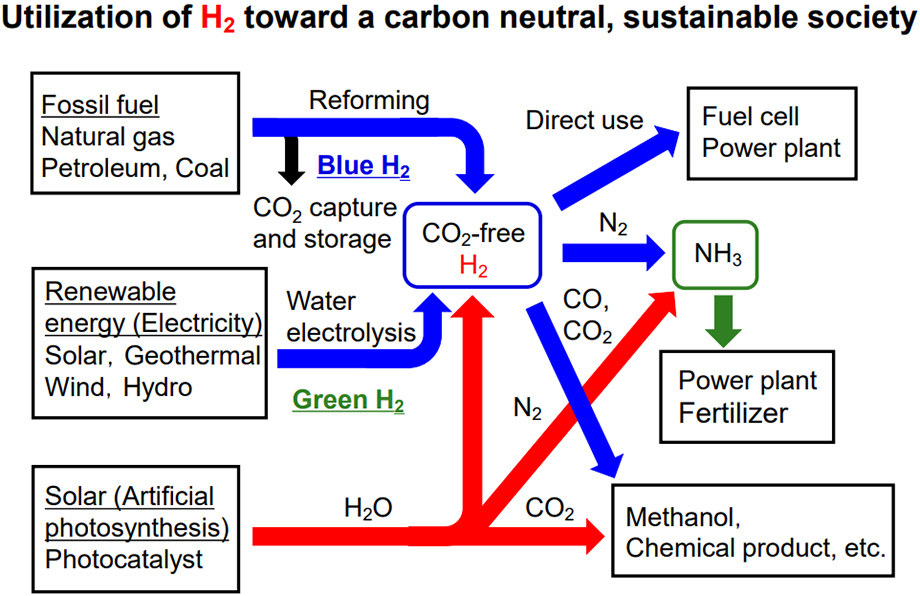
Although it will take a long time to reach this point in terms of technology, we are aiming to implement the technology of artificial photosynthesis as a means of producing green hydrogen. Finally, if we can find a route to making things directly from water, we will be much closer to solving energy and environmental problems.
* The information contained herein is current as of February 2024.
* The contents of articles on Meiji.net are based on the personal ideas and opinions of the author and do not indicate the official opinion of Meiji University.
* I work to achieve SDGs related to the educational and research themes that I am currently engaged in.
Information noted in the articles and videos, such as positions and affiliations, are current at the time of production.

