Redox flow batteries for addressing renewable energy challenges
Renewable energy, derived from natural sources such as sunlight, does not require fuel; however, it has inherent limitations in its power generation capacity.
Japan has already built large hydroelectric power plants wherever feasible, making further reliance on hydropower impractical. Wind power generation is feasible only in windy regions, which are scarce in Japan. Solar power is the only renewable energy source that can be utilized nationwide. However, its output depends on weather and seasonal changes, and it does not generate electricity at night. Since power generation from renewable energy sources is not always stable, effective energy storage technologies are essential for its widespread adoption.
One of the storage technologies is pumped hydropower. This system uses two reservoirs: an upper reservoir located at a higher height and a lower reservoir at a lower height. When there is surplus electricity, water is pumped from the lower to the upper reservoir. When there is a shortage of electricity, water is released from the upper to the lower reservoir to generate electricity. In recent years, solar power has been used to pump water during the day, with the stored water released to supply electricity at night. However, Japan has limited terrain suitable for constructing pumped storage power plants, making new installations nearly impossible.
With the increasing adoption of renewable energy, new storage technologies are needed to efficiently store and use electricity. Among these, the redox flow battery, on which we are conducting research, has attracted significant attention.
A redox flow battery is a type of energy storage device that stores electrical energy in liquids containing active materials. Its defining feature is the decoupling of the tanks that store energy from the cell where charging and discharging proceed.
Lithium-ion batteries, another type of energy storage device, cannot decouple energy storage capacity from charging/discharging functions within a single cell. This means their charging and discharging capacity is limited by the amount of lithium active materials incorporated during manufacturing. To increase capacity, the only option is to connect additional batteries, which can lead to excessive capacity or charging/discharging rates. Furthermore, as shown in incidents like mobile phone battery fires, lithium-ion batteries pose significant safety management challenges. Deploying these batteries on an industrial scale necessitates comprehensive fire prevention systems.
In contrast, redox flow batteries allow the size of the tanks and the cell to be designed independently. The cell refers to the discharging and charging unit where electrolytes flow and oxidation-reduction (redox) reactions occur at the electrodes. Increasing the size of the tanks and the amount of electrolyte enables greater electricity storage capacity. Enlarging the surface area of the electrodes within the cell increases power output. Unlike lithium-ion batteries, redox flow batteries use water-based electrolytes, eliminating the risk of fire and enhancing safety.
The amount of renewable energy that can be stored and the required output levels vary among power plants nationwide. However, redox flow batteries can be customized to suit local environmental and operational conditions, enabling optimal system design.
Designing batteries with reduced internal resistance
A redox flow battery consists of a cell for charge/discharge, two tanks, electrolytes in which active materials proceeding redox reactions are dissolved, pumps, and other components.
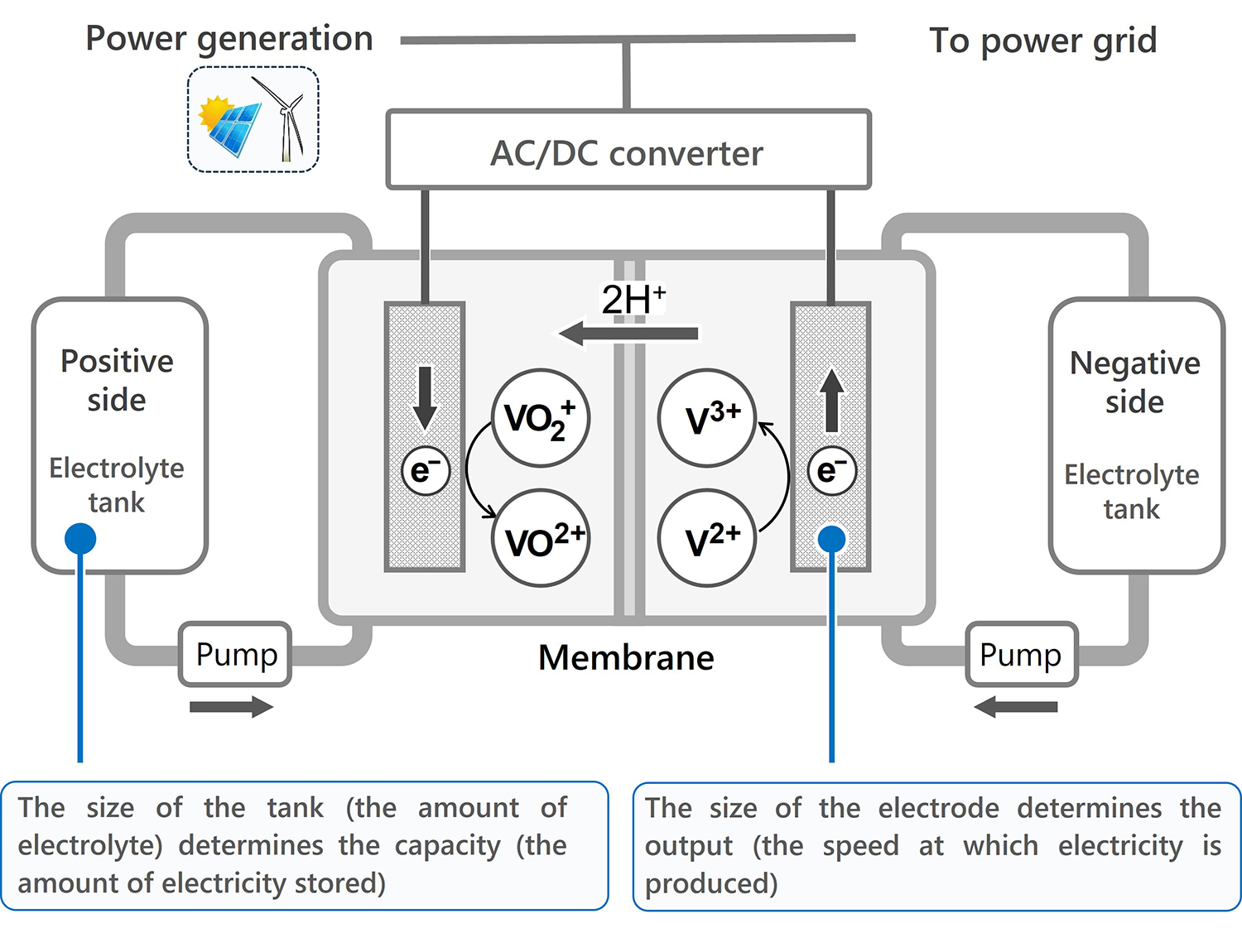
Generally, the active materials proceeding the redox reactions is vanadium, the liquid is an aqueous solution of sulfuric acid, and the electrodes are carbon porous sheets. As the oxidation state of vanadium changes at the carbon electrodes, charges are transferred at the electrodes. This charge transfer generates an electric current, which is then converted by an AC/DC converter and transmitted to power networks.
Currently, redox flow batteries have only been introduced in a limited number of locations, such as facilities of Hokkaido Electric Power and Kashiwazaki City in Niigata Prefecture. Our research group is actively collaborating with the National Institute of Advanced Industrial Science and Technology (AIST), other universities, and companies to promote the social deployment of redox flow batteries.
One of the hurdles to deploying redox flow batteries in society is their high internal resistance. Charging and discharging a battery always entails some internal resistance – the resistance to electron/ion conduction, charge transfer reaction, and active material transport caused by the battery’s internal materials and structure. Consequently, the stored electrical energy cannot be discharged at 100% efficiency, resulting in some energy loss.
One cause of increased internal resistance is a slow reaction rate. To address this issue, electrodes are chemically treated to facilitate the redox reactions of vanadium. In addition, active materials transport slowly in the liquid, and evenly distributing vanadium within the electrolytes is challenging. These difficulties in reaction kinetics and material transport require additional voltage drops, which increase energy loss. Since this lost energy is wasted, it poses challenges for the practical application of redox flow batteries.
To address these drawbacks, we are working to enhance the performance of the carbon sheets used as electrodes. We create pores, approximately 0.01 mm in diameter, in the carbon material to improve electrolyte flow. Making these pores more uniform allows the electrolyte to flow more evenly. Additionally, increasing the heating temperature during the crystallization treatment of the carbon sheets has been shown to improve crystallinity, resulting in a higher current output per unit area. We are refining the manufacturing methods and shapes, continuously iterating through design and experimental cycles to achieve the optimal structure.
Contribution of deploying redox flow batteries to energy self-sufficiency
The cost presents an even greater challenge to the widespread deployment of redox flow batteries. Currently, redox flow batteries cost three to four times as much as lithium-ion batteries to store the same amount of electricity.
For renewable energy businesses, solar panels and wind turbines are the primary capital costs, while batteries are considered a secondary expense. Since redox flow batteries are currently prohibitively expensive, most businesses use lithium-ion batteries instead when necessary.
The cost of a redox flow battery is approximately divided equally among three components: the electrolytes, the cell, and the tanks and pumps. Unless the cost of each component is reduced to about one-third, redox flow batteries will not be able to compete with lithium-ion batteries.
Vanadium, the raw material, is inherently expensive. Widely used in steel materials, particularly for reinforced concrete, its price tends to spike as construction demand increases. From a technical perspective, processing carbon materials to accelerate reaction rates also adds to the cost. Moreover, fluorine-based materials used in ion exchange membranes are not only costly but may also pose environmental risks, requiring the development of fluorine-free membranes.
More widespread adoption of redox flow batteries would enable us to utilize more electricity generated from renewable sources with less waste, bringing us closer to achieving carbon neutrality by 2050. Beyond energy storage, redox flow batteries could also serve as emergency power supplies during disasters, providing electricity to evacuation centers. In fact, when an earthquake struck Hokkaido, the redox flow batteries remained intact and continued to charge/discharge without interruption.
Japan, which relies on imports for oil, coal, and natural gas, pays a significant portion of its national wealth to other countries. Increasing energy self-sufficiency through solar and other renewable energy sources would enhance security and prevent the outflow of national wealth. To enrich Japanese society and improve people’s lives, I am committed to advancing research toward the practical application and widespread adoption of redox flow batteries.
* The information contained herein is current as September 2025.
* The contents of articles on Meiji.net are based on the personal ideas and opinions of the author and do not indicate the official opinion of Meiji University.
* I work to achieve SDGs related to the educational and research themes that I am currently engaged in.
Information noted in the articles and videos, such as positions and affiliations, are current at the time of production.

